






| Availability: | |
|---|---|
| Quantity: | |
Product name | Scleral Plug Forceps | |||
Material | Stainless Steel | |||
Feature | Reusable/Dull Finish | |||
Description | For holding scleral plugs during insertion and removal. Overall length 105mm. | |||
Properties | Ears, Eyes, Nose and Throat Surgical Instruments | |||
Selling Units | Single item | |||
Single packaging size | 19.5cm*7cm*4cm | |||
Single gross weight | 0.07 kg | |||
Lead Time | 1 week upon upfront payment ( 1~10pcs ) ,4 weeks upon unfront payment ( 10~50pcs ) | |||
Scleral Plug Forceps IF-4300 For holding scleral plugs during insertion and removal, Overall length 105mm. |
| ABOUT PRODUCTSMicro instrument mainly applied in ophthalmic and ENT surgery, require the most precise mechanical treatment as well as surface treatment. We had developed all necessary capability and modernized equipment to design and manufacture both reusable and disposable micro instrument. |
—— ODM/CUSTOMIZED SERVICES Providing full cooperation to realize your design and creativity | —— OEM SERVICES 8 years experience in OEM project, we use APQP management system to ensure customer satisfaction of delivery. | —— MAINTENANCE SERVICE Regular maintenance work is important for reusable precise instruments, contact us at anytime to help you take good care of your tools. |

BELLE HEALTHCARE
————— + —————
About our company, production process and advantages
 Belle Healthcare Technology Co., Ltd. provides highest quality micro instruments for ophthalmic community. Original founded in 2009 named Belle in Focus as an OEM manufacturer for customers in Europe and USA (North America). The company was reorganized, and name was changed in 2017 to expand its profile to disposable instruments, ENT instruments and consumable products. In China, Belle Healthcare is recognized as a full-service brand due to its customized designing capability, advanced R&D equipment, direct salesforce and after-sale service. For international market, Belle Healthcare has established distribution channel for customers in Europe and Southeast Asia. |
—— Mission Focus on standardization and precision of microsurgery | —— Vision To become a reliable brand of microsurgery instruments over the world | —— Core Value · Customer Orientation · Craftsmanship · Continuous Improvement · Technology Inheritance · Return On Contribution |
————— + —————
About how to provide high-quality surgical instruments
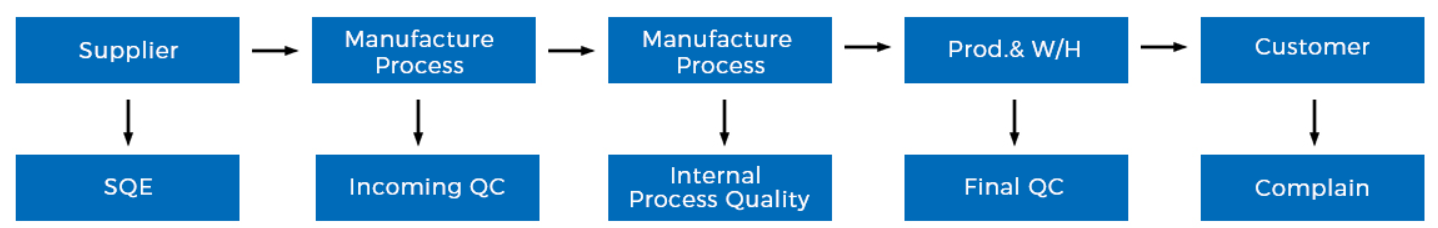
For 11 years, hospitals and medical institutions have trusted us to provide high-quality surgical instruments that meet the stringent standards of surgeons. Not only is our ability to develop and produce new and desirable surgical instruments such as ophthalmology, but our surgeon satisfaction is assured -- our team of experienced surgical instrument specialists saves time and money for thousands of organizations and individuals each year.
————— + —————
Our company's advantages
|
|
———————————————————— + ————————————————————
content is empty!